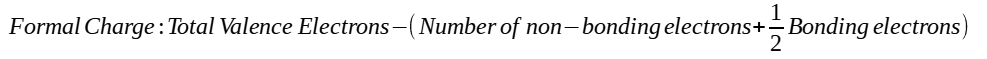- To acquire lower energy state & higher stability
- To follow octet rule or to achieve the configuration of Noble Gases, all other atoms lose/gain/share (equally/unequally) their electron(s), and make different types of bond with same or other atoms
Hello, This is my second post here.
Chemical Bonding and Molecular Structure
Why do atoms combine together?
# Types of Bonds:
- Ionic Bond: Also known as Electrovalent Bond
- Forms between metals and non-metals
- By transfer of electron from metal to non-metal
- Covalent Bond:
- Formed by two non-metals
- By equal sharing of one or more electrons
- Co-ordinate Bond: Also known as Dative Bond
- Type of covalent bond
- Formed between two non-metals
- By unequal sharing of electron(s) and this sharing of electron(s) is provided by any of the bonded atom
# Covalent Bond:
- Types of Bonds:
- Single Bond (σ bond)
- Multiple Bonds:
- Double Bond (1 σ & 1 π bond)
- Triple Bond (1 σ & 2 π bond)
# Important:
- Bonded electrons: Bond Pairs
- Non-bonded electrons: Lone Pairs of electron
# Co-ordinate Bond:
- It is formed when in any of the two atoms, one has completely filled octets and has one or more lone pair of electrons and the second atom is just short of two electrons to complete its octet
- It is a type of covalent bond & is replaced as:
- In first & second period elements, co-ordinate bond is replaced into covalent bond by
"formal
charge"
- Beyond the second period element, co-ordinate bond changes into covalent double-bond
# Valency
- The combining capacity of an atom in a molecule is called valency & is determined by valence electrons
- Valency with respect to Hydrogen:
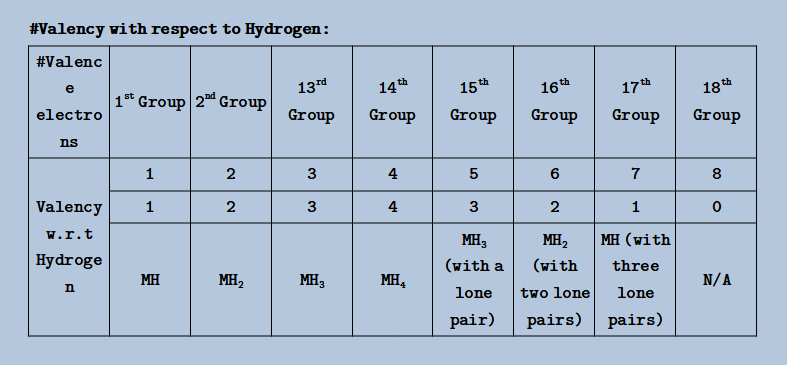
- # Periodicity: (Hydride)
- Along a Period: Acidic Strength Increases & Basic Strength Decreases
- Down a Group: Acidic Strength Increases & Basic Strength Decreases
- Most acidic hydride: HI
- Valency with respect to Oxygen:
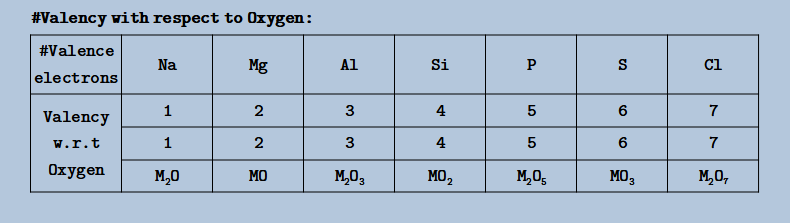
- # Periodicity: (Oxides)
- Metal oxides are basic & non-metal oxides are acidic in nature
- Along a Period: Acidic Strength Increases & Basic Strength Decreases
- Down a Group: Acidic Strength Decreases & Basic Strength Increases
- Acidic Strength of Non-metals ∝ E. N. or ∝ Oxid. Number
# Covalency:
- It is the number of co-valent bonds formed by an atom in a molecule
- For 1st & 2nd Period elements: Covalency = No. of unpaired valence electrons in excited state
- Beyond 2nd Period elements: Covalency = No. of unpaired valence electrons in ground as well as excited state
Structure of Compounds
# Rules:
- Write the central atom: Central atom: That atom works as a central atom which makes maximum number of covalent bonds
- Hydrogen is placed at terminals with O-H bonds & number of O-H bonds is equal to basicity of the
compound
- Basicity: Number of Hydrogen atoms in compound, except:
- H3PO3 = 2
- H3PO2 = 1
- H3BO3 = 1
- Basicity: Number of Hydrogen atoms in compound, except:
- If “per” word is used in the name of the compound, then per-oxide bond (-O-O-) must be present and should be directly attached to the central atom
- Reducing Hydrogen: When Hydrogen is bonded with any atom and that atom is attached with a more electro-negative atom (MENA), then that Hydrogen is known as “Reducing Hydrogen”
# Some important acids:
- H2SO3: Sulphurous Acid
- H3PO4: Ortho-phosphoric Acid
- H3PO3: Phosphorous Acid
- H2SO5: Caro's Acid or Peroxo-mono-sulphuric Acid
# Different types of Acids:
- #1 Pyro Acids:
- 2 Mole of Acid (B >= 2) (-H2O) → Pyro Acid
- #2 Meta Acids:
- 1 Mole of “ic” Acid (-H2O) → Meta Acid
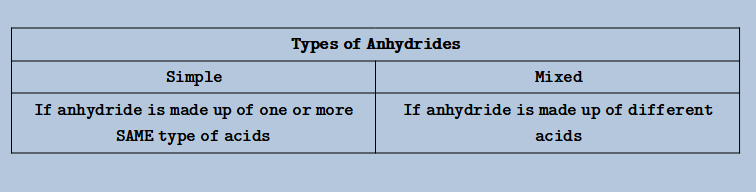
- #3 Acid Anhydride:
- Removing all the Hydrogens (in whole numbers) gives the anhydride of that acid
VALENCE BOND THEORY
- Given by: Pauling & Heitler
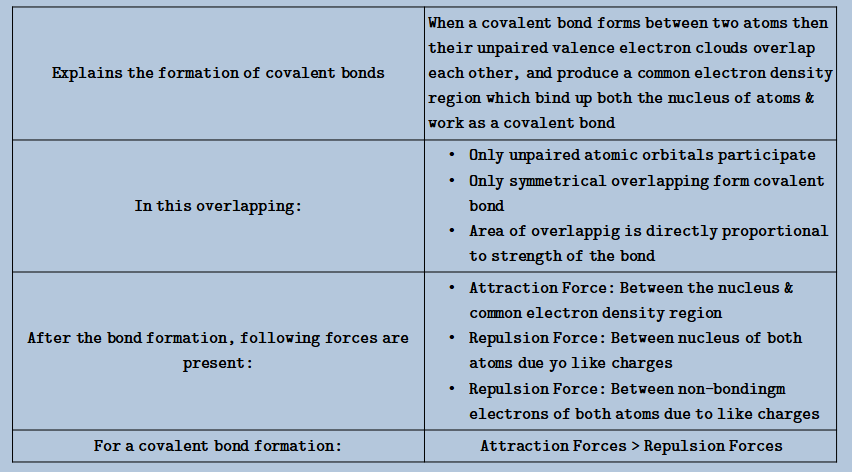
# Types of Overlapping:
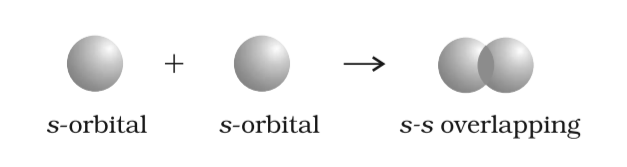
- s-s overlapping:
- In any axis:
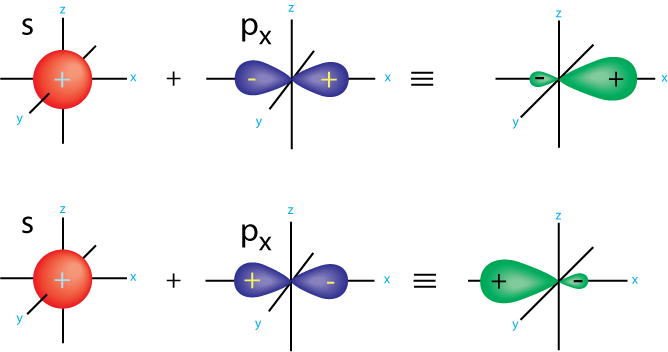
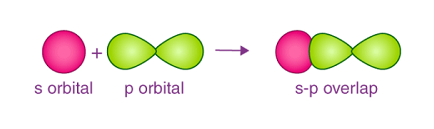
- s-p overlapping:
- In the axis same to that of the p-orbital:
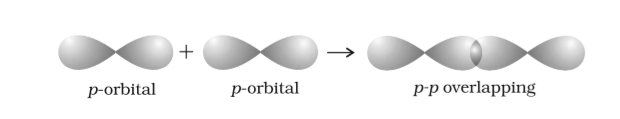
- p-p overlapping:
- head-to-head overlapping:
- Coaxial:
- headside-by-side overlapping:
- Lateral:
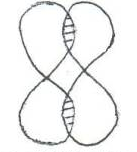
- head-to-head overlapping:
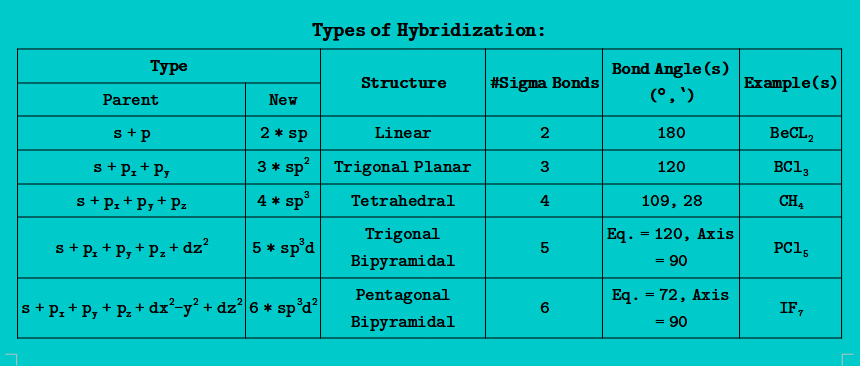
HYBRIDIZATION (Mixing of Atomic Orbitals):
- When two or more Atomic orbitals are mixed then same number of new orbitals are formed (which have same energy, size, and shape) and are called hybrid orbitals and this phenomena is called as hybridization
- A hybrid orbital can be represented as:
- In a molecule, these hybrid orbitals are present around the central element in such a way that they feel minimum repulsion and provide shape to the molecule which is called regular geometry
- According to Valence Bond Theory: Hybridization = Number of σ Bonds
- Number of σ Bonds = ( Number of atoms + n) -1; n = Number of cycle or ring
Total Valence Shell e Method:
If (TVSe =< 8):
Hybridization = (TVSe/2)
elif (TVSe> 8):
Hybridization = (TVSe/8)
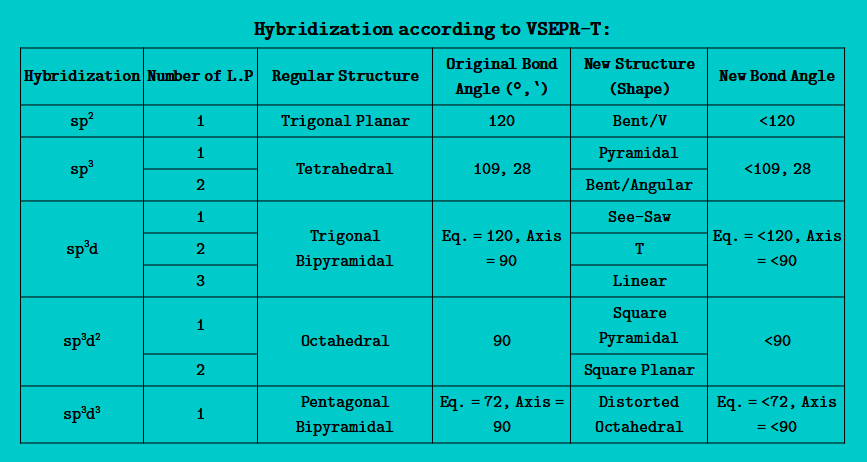
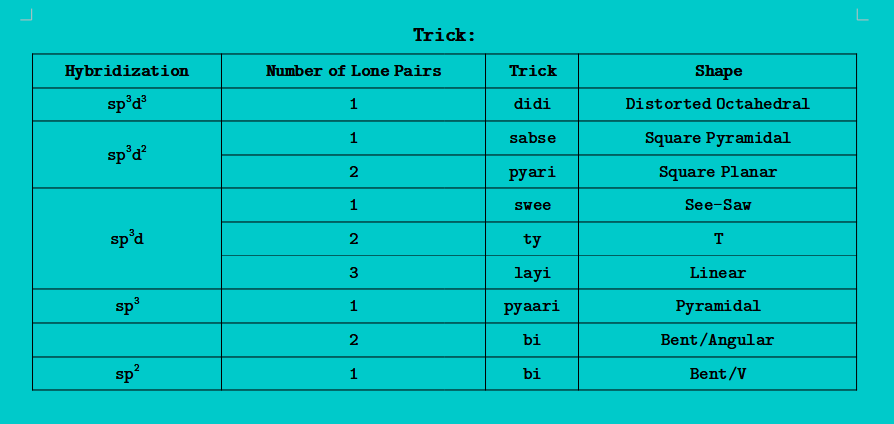
Valence Shell Electron Pair Repulsion Theory (VSEPR-T):
- In a molecule, when atoms= have one or more lone pair of electron(s), then lone pair of electrons repel the lone pair of electron or bond pair of electron and destroy the geometry of the molecule, and reform the geometry which is called irregular geometry or shape
- In such molecule(s), following forces are present:
- L.P - L.P Repulsion
- L.P - B.P Repulsion
- B.P - B.P Repulsion
- Hybridization = Number of σ Bonds + Number of L.P of electrons